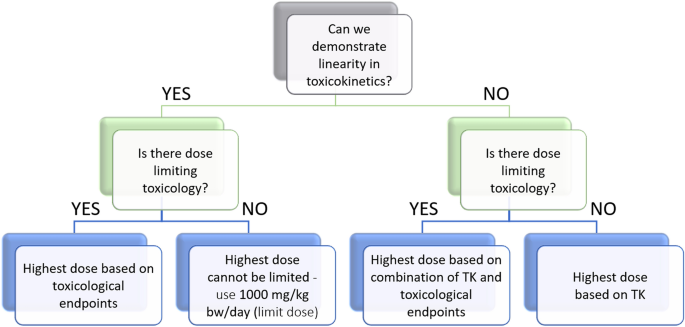
Preliminary Assessment of Acute and 28-Day Repeated Dose Oral Toxicity of a Newly Developed Herbal Mixture on Experimental Anima
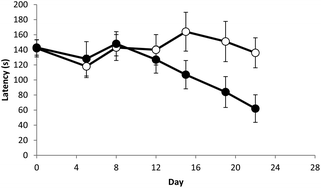
Functional assessments in repeat-dose toxicity studies: the art of the possible - Toxicology Research (RSC Publishing)

Single- and repeated-dose 28-day oral toxicity study of MDM hydantoin in Sprague–Dawley rats | SpringerLink

Repeated Dose Toxicity Study and Developmental and Reproductive Toxicology Studies of a Respiratory Syncytial Virus Candidate Vaccine in Rabbits and Rats - Alan H. Stokes, Kelle Franklin, Daniel E. Fisher, Lorraine M.

Analysis of repeated dose toxicity studies. The study protocols and... | Download Scientific Diagram

180 Day Repeated-Dose Toxicity Study on Forchlorfenuron in Sprague–Dawley Rats and Its Effects on the Production of Steroid Hormones | Journal of Agricultural and Food Chemistry

Repeated-doses and reproductive toxicity studies of the monoterpene 1,8-cineole (eucalyptol) in Wistar rats - ScienceDirect
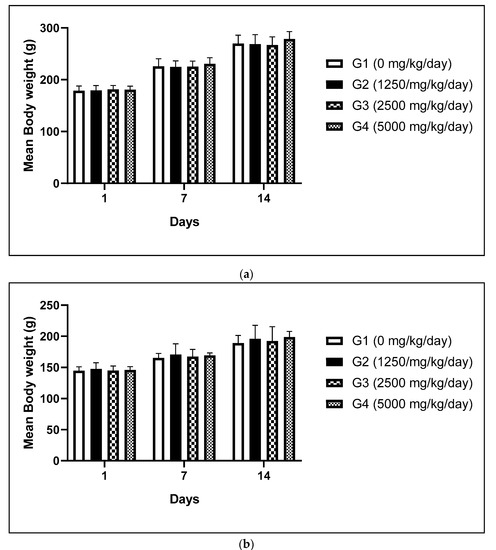
Foods | Free Full-Text | Evaluation of Subchronic Oral Dose Toxicity of Freeze-Dried Skimmed Powder of Zophobas atratus Larvae (frpfdZAL) in Rats

Identification of repeated dose toxicity studies described in 88 safety... | Download Scientific Diagram
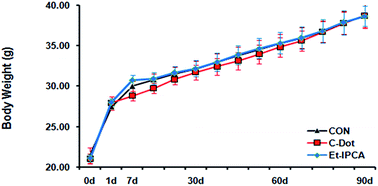
Single and repeated dose toxicity of citric acid-based carbon dots and a derivative in mice - RSC Advances (RSC Publishing)
Evaluation of 90 day repeated dose oral toxicity and reproductive/developmental toxicity of 3'-hydroxypterostilbene in experimental animals | PLOS ONE