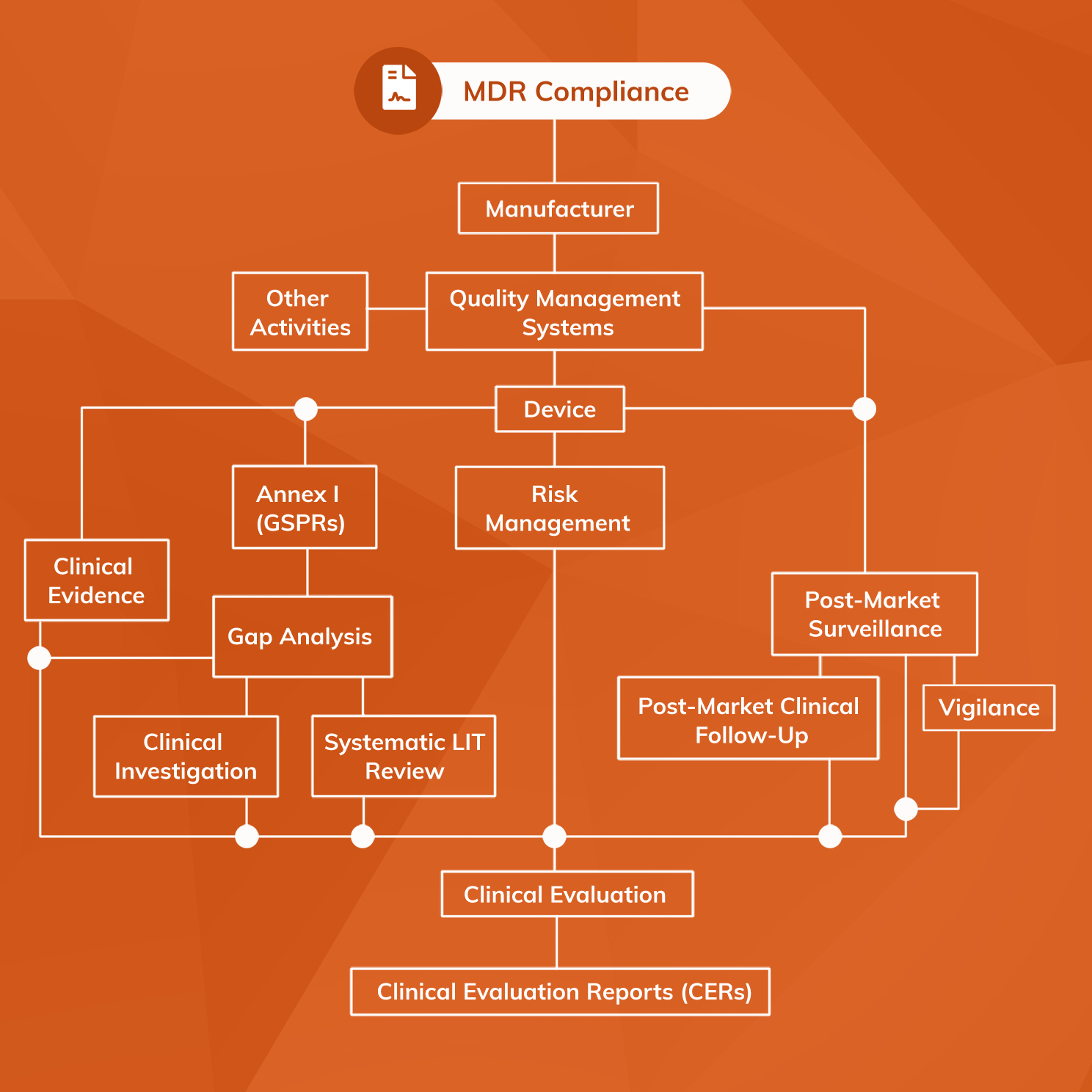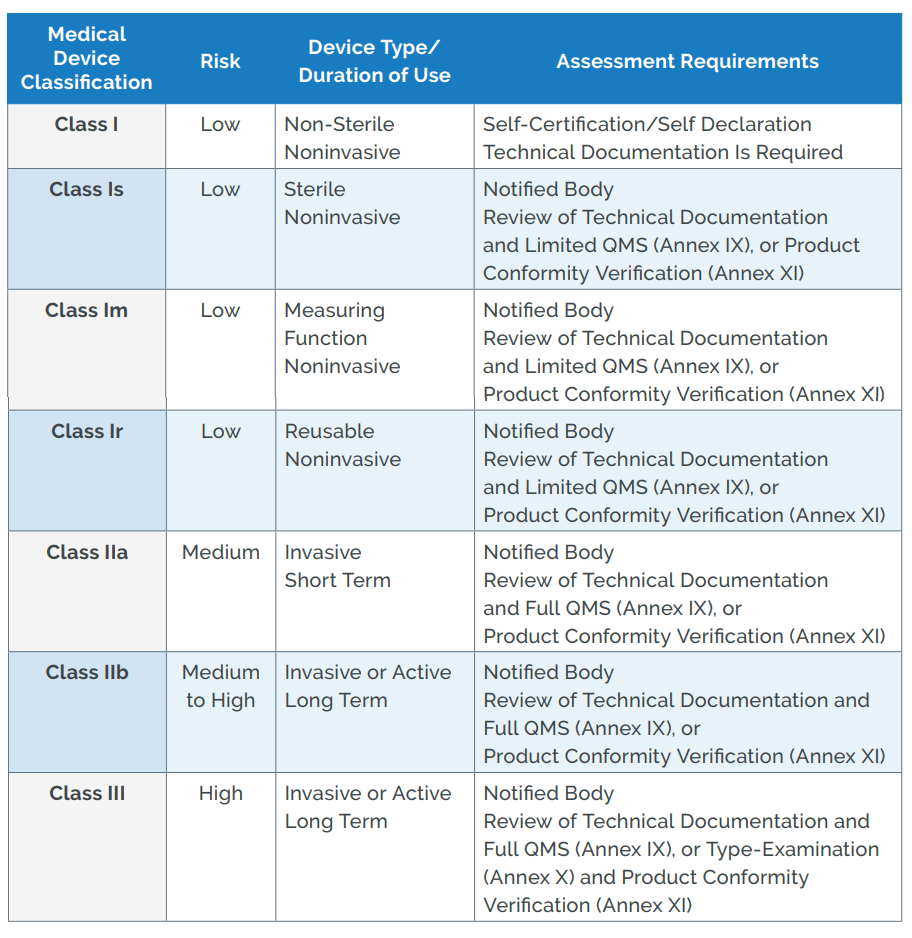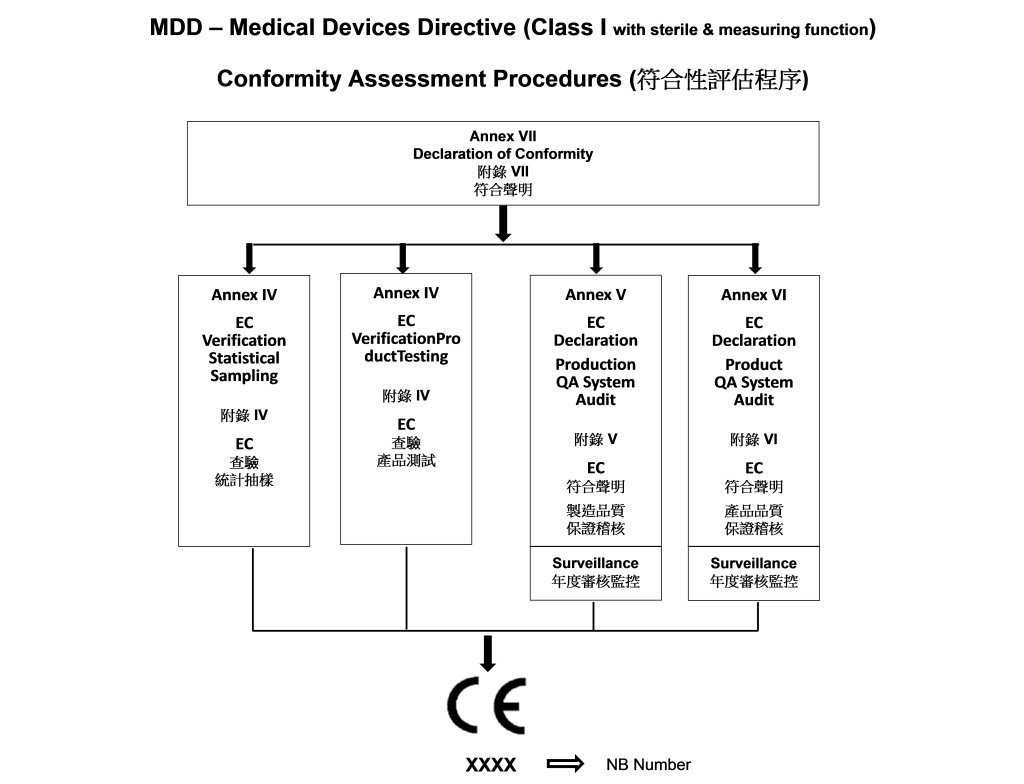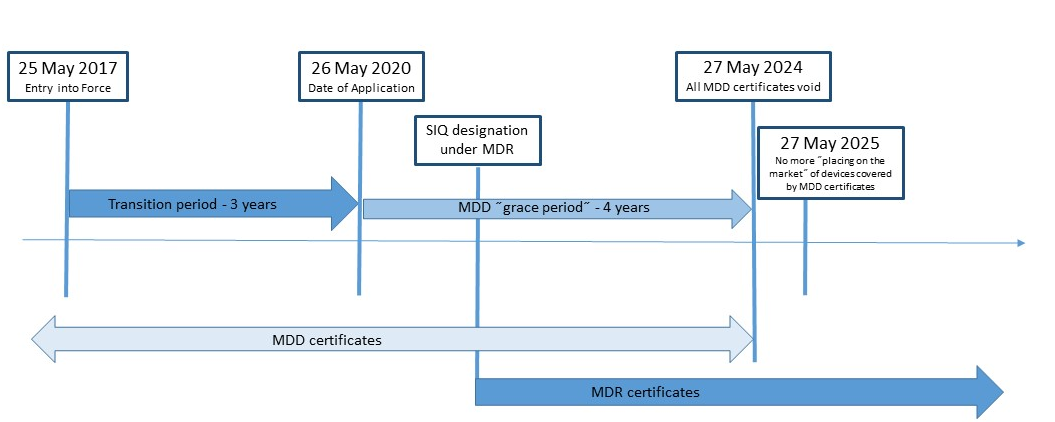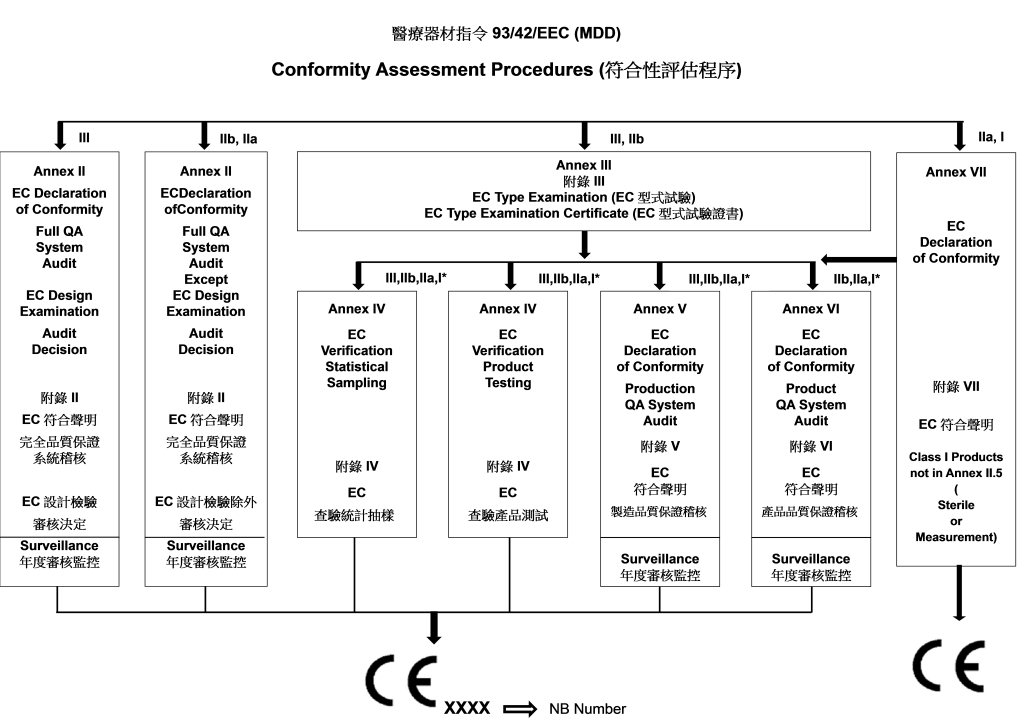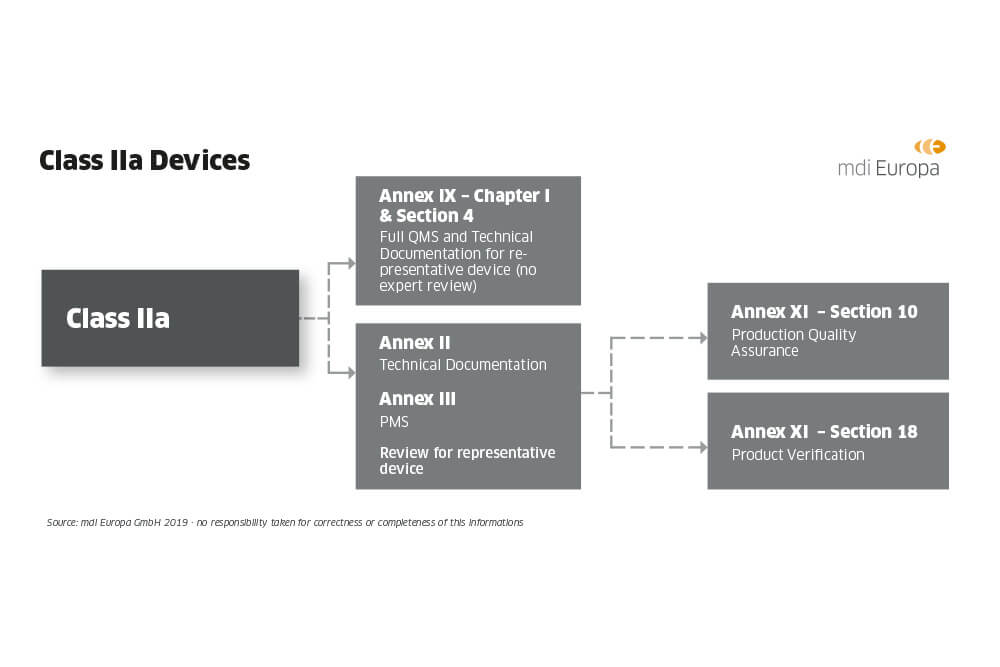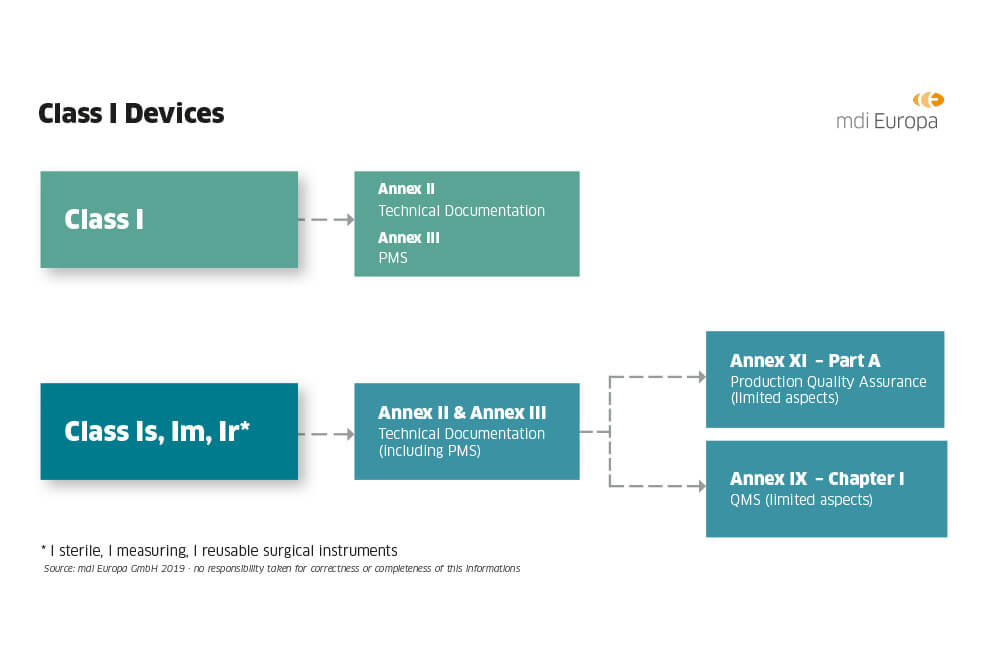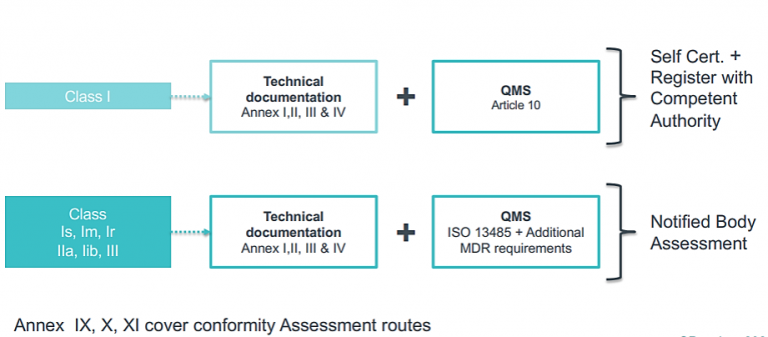
CE Marking a Medical Device under the EU MDR | Wellcome / EPSRC Centre for Interventional and Surgical Sciences - UCL – University College London
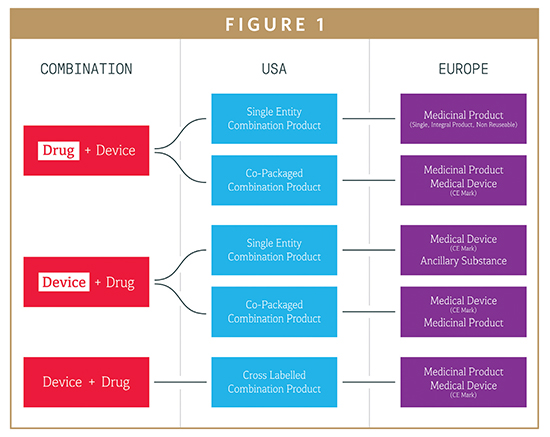
DEVICE REGULATIONS - The New Medical Device Regulation & the Applicability of Article 117 to Medicinal Products

How are medical devices regulated in the European Union? - Elaine French-Mowat, Joanne Burnett, 2012
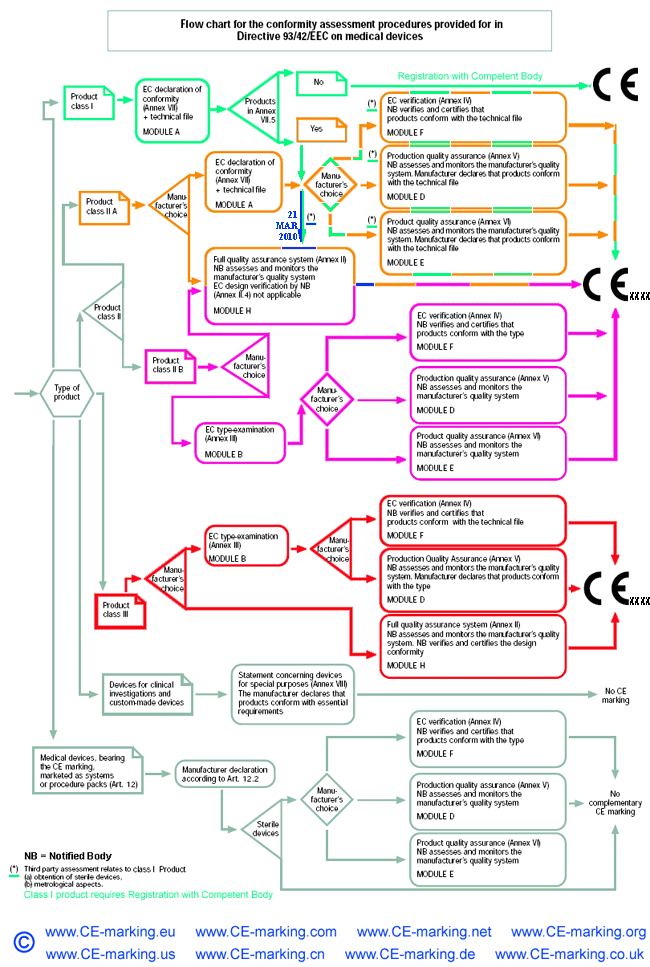

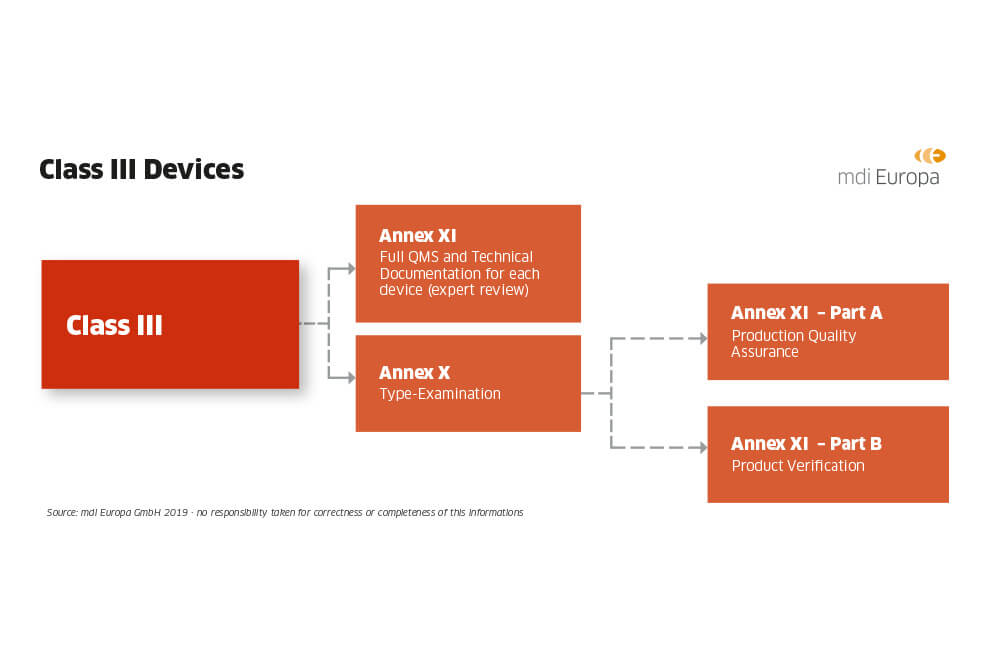
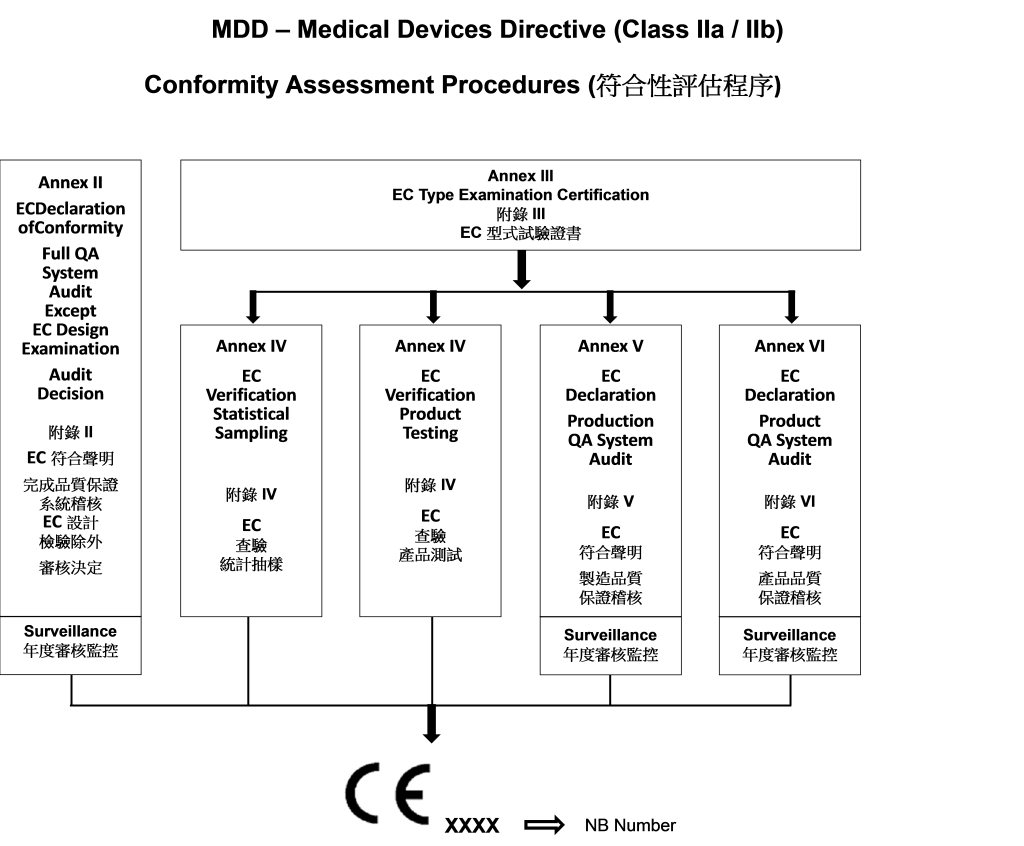
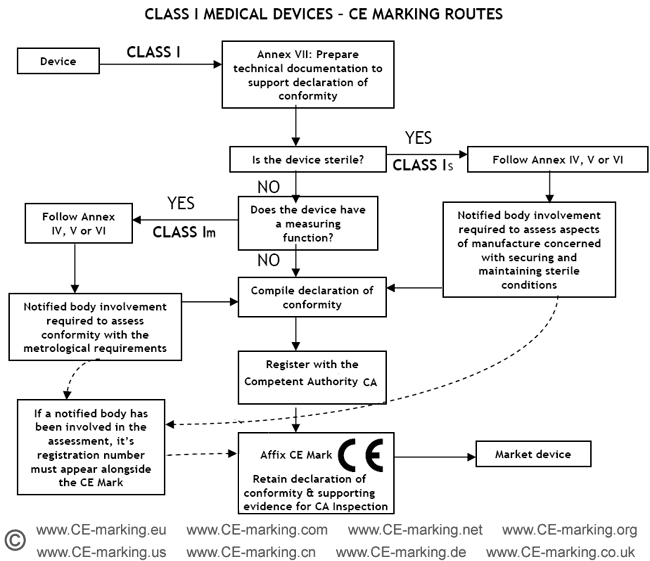

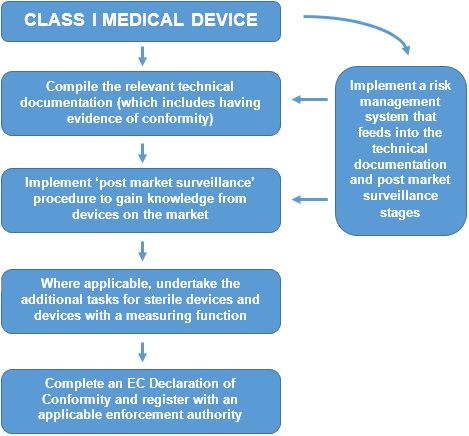
![EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast](https://x6t6s6a2.rocketcdn.me/wp-content/uploads/2021/08/class-IIb-medical-devices.jpeg)